Research
Research Focus
Respiratory chain
In mitochondria ATP is regenerated by a process called oxidative phosphorylation. It involves a series of protein complexes known as the respiratory chain that collectively, transfer electrons and pump protons across the mitochondrial inner membrane that will be used to fuel ATP synthesis. The precise organization of these individual respiratory complexes (commonly called complexes I to V) within the inner mitochondrial membrane was (and still is) debated.
In our team, we study the organization of these complexes in mitochondria. Using cutting-edge cryo-electron tomography, our team has shown how these complexes organize in their native cellular environment. The respiratory complexes assemble into larger, stable structures called “supercomplexes” or “respirasomes”. We revealed that these supercomplexes are not randomly distributed but rather organized in specific membrane regions of mitochondria.
Our current research aims to answer several critical questions: What is the precise role of these supercomplexes in mitochondrial function? Do they actively shape the mitochondrial membrane, notably cristae? Do they provide a functional advantage, such as optimizing electron flow or minimizing energy loss? How has their organization and diversity evolved across different species, particularly in photosynthetic organisms where mitochondrial function must be coordinated with chloroplasts?
Research Focus
Gene expression
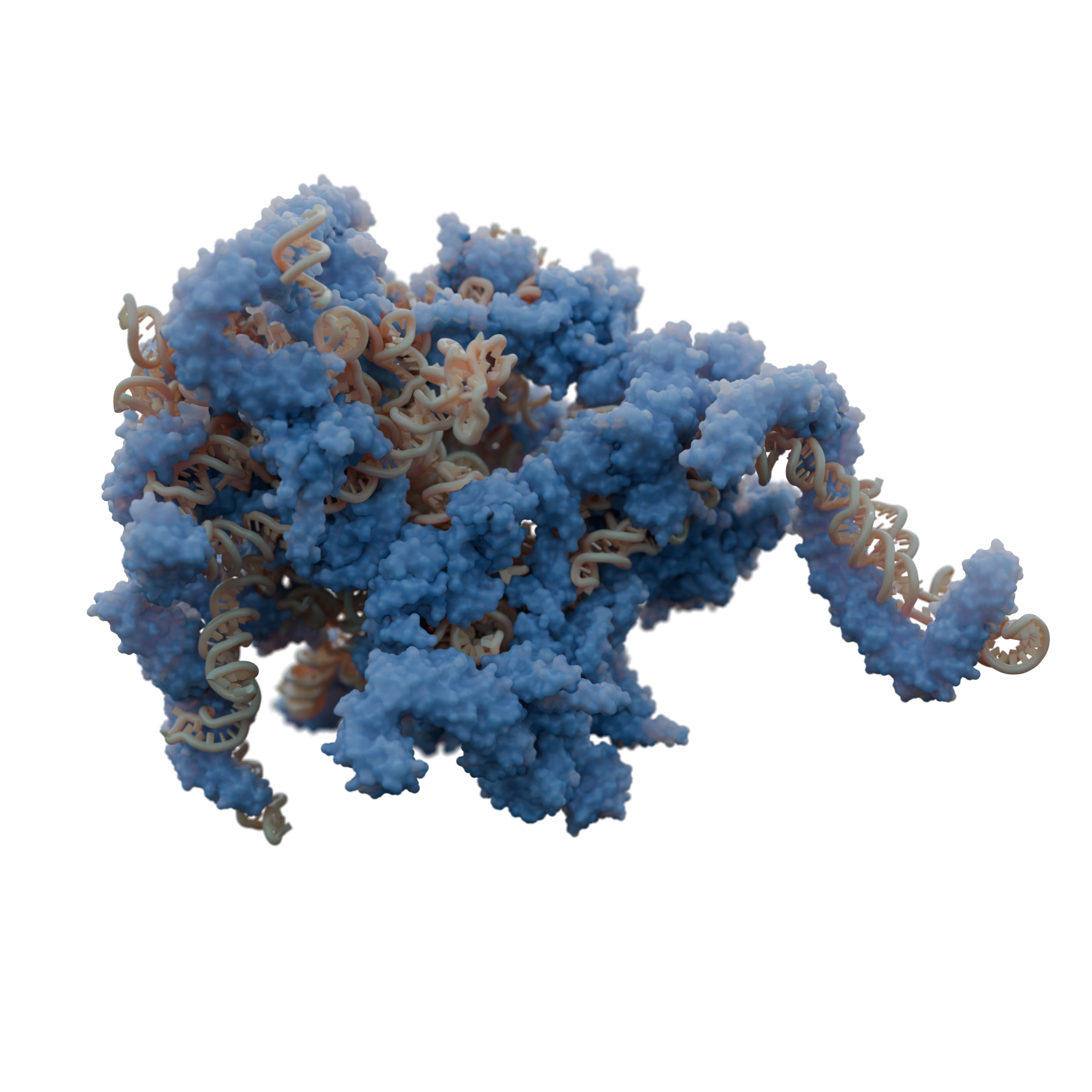
Mitochondria possess a dedicated protein synthesis machinery that involves specialized ribosomes, called mitoribosomes. They translate the few essential proteins encoded by mitochondrial DNA, primarily components of the respiratory complexes.
Using cryo-electron microscopy, we have contributed significantly to describing the unique and diverse structures of mitoribosomes across various species, such as mitoribosomes with expanded rRNAs in flowering plants, extremely reduced rRNAs in trypanosoma, as well as fragmented rRNAs in green algae. Furthermore, using cryo-electron tomography, we have shown how membrane-bound specialization of translation occurs within mitochondria.
We are particularly interested in understanding the types of translational regulation involved during metabolic changes, and how mitochondrial translation is coordinated with the assembly of respiratory complexes. What roles do mitochondria-specific ribosomal proteins play? How do unique structural features contribute to their specialized function?
Research Focus
What are mitochondria?
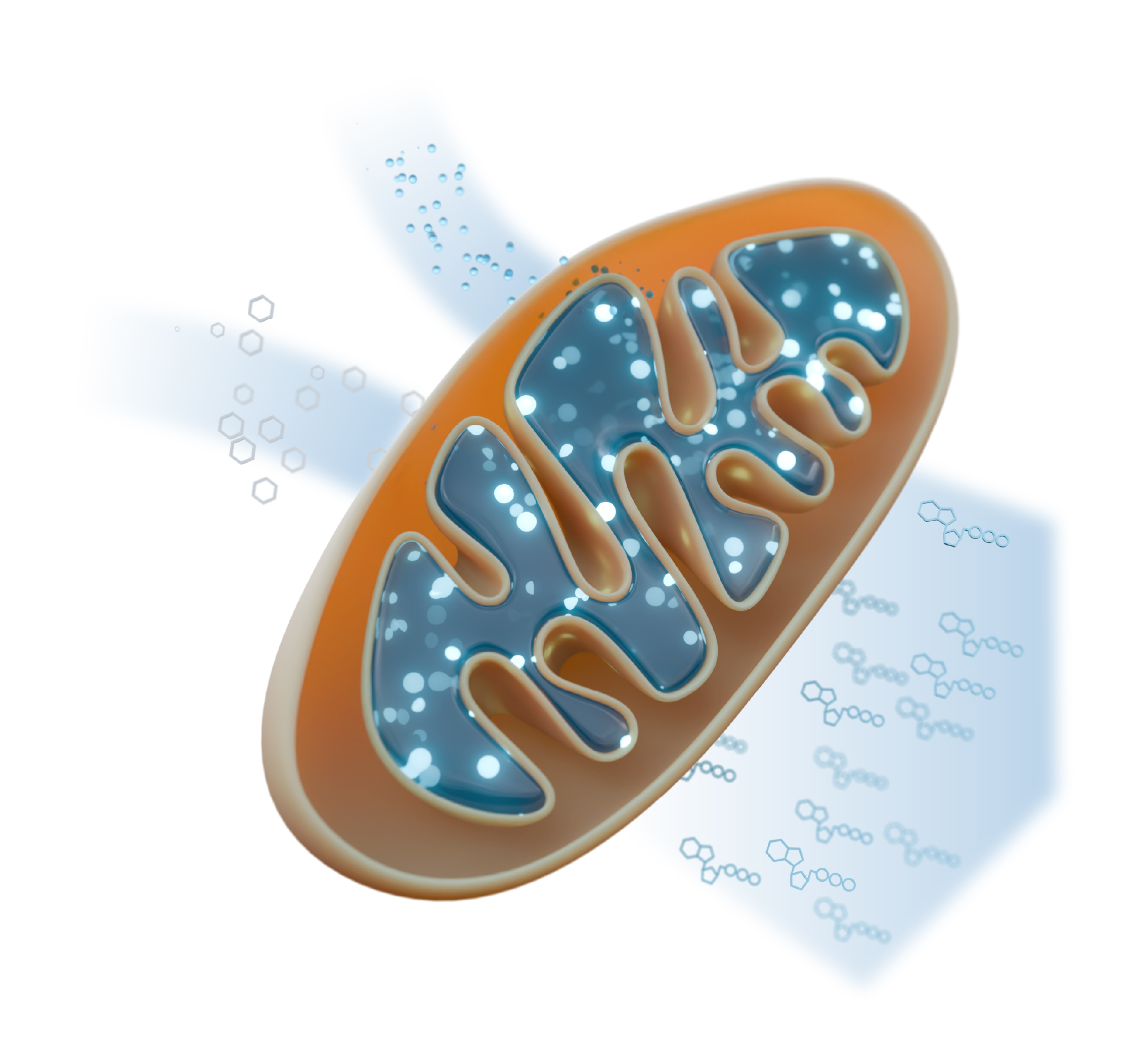
Mitochondria are tiny, membrane‐bound “power stations” found in every complex organism (plants, fungi, humans, and more). Their main job is to regenerate the cell’s energy currency, ATP. Think of ATP as the coin you need to power virtually every reaction in the cell.
Inside mitochondria, food molecules (like sugars and fats) are broken down, releasing electrons. These electrons travel through a series of protein complexes sitting in the mitochondrial membrane, called the respiratory chain, which uses their energy to pump protons and build an electrochemical gradient. ATP synthase, another membrane-embedded enzyme, harnesses this proton gradient to regenerate ATP. Oxygen, the very oxygen we breathe, serves as the final electron acceptor in this chain, making mitochondria the site where the food you eat and the air you breathe combine to keep your body running.
Because ATP powers almost every cellular process, mitochondrial dysfunction can have devastating effects. In humans, defects in mitochondrial function underlie a range of disorders from muscle weakness (myopathies) to nerve degeneration (neuropathies).
Mitochondria are remarkably complex and can be seen as a “cell within a cell.” They carry their own circular genome (mitochondrial DNA), gene expression machinery, etc. This autonomy reflects their origin: nearly two billion years ago, a simple single-celled organism (called archaea) swallowed a bacterium but didn’t digest it. Instead, the two teamed up, the bacterium provided extra energy, and its host offered protection (a process known as endosymbiosis). Over time, that former free-living bacteria evolved into today’s mitochondria, giving the cell more energy than primitive cells could ever manage alone. That partnership kick-started the rise of all complex life, plants, fungi, animals, and ultimately us (collectively known as eukaryotes), by allowing cells to grow bigger, specialize, and work together in ways that simple cells never could.
In other words, studying mitochondria allows us to look back in time at the very beginning of complex life.

Research Focus
Looking at molecules using cryo-electron microscopy
Cryo-electron microscopy (cryo-EM) and cryo-electron tomography (cryo-ET) are two powerful techniques that let scientists “freeze” tiny biological machines, like proteins, or entire cells and image them using electrons. By using electrons rather than light, like in classical light microscopy, we can reach much higher resolution and resolve things with greater details.
In cryo-EM, researchers first purify a single type of molecule (for example, an enzyme, or a virus) using classical biochemistry techniques. The purified sample is plunged into liquid ethane cooled to around –184 °C. This vitrifies the water into glass-like ice in milliseconds, trapping each molecule in its native shape without forming ice crystals. The frozen sample is inserted into an electron microscope, where a beam of electrons (thanks to its much shorter wavelength compared to visible light) can resolve details down to nearly individual atoms. By collecting tens of thousands to millions of two-dimensional projection images, computers then align and average these snapshots to build a 3D picture (called “3D map”) revealing the molecule’s architecture with near-atomic precision.
Cryo-ET uses the same rapid-freezing strategy but tackles intact cells or tissue instead of isolated molecules. Because whole cells are too thick for electrons to penetrate, researchers first need to thin the frozen specimen into electron-transparent slices (just a few hundred nanometers thick!). Inside the microscope, the sliced cell is tilted back and forth, taking a series of images from different angles, like a CT scan in a hospital. Specialized software back-projects these images into a 3D volume (called “tomogram”), showing how proteins and complexes are arranged and interact within their crowded, native environment, along membranes, filaments, and organelles.
In short, cryo-EM delivers ultra-sharp 3D pictures of purified molecular machines, while cryo-ET provides volumetric views of those same structures in the wild world of the cell. Together, they let us see both the detailed shapes and the real-life “street map” of biology at the tiniest scales.